When A System Is At Equilibrium Spontaneous
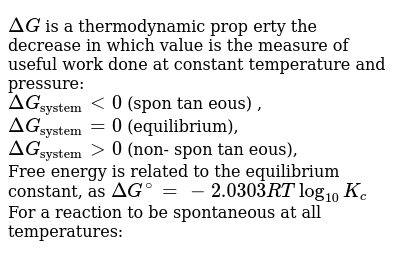
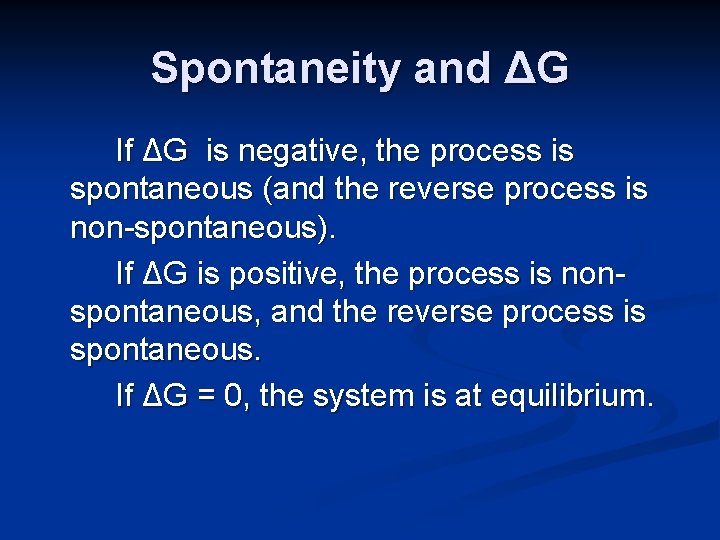
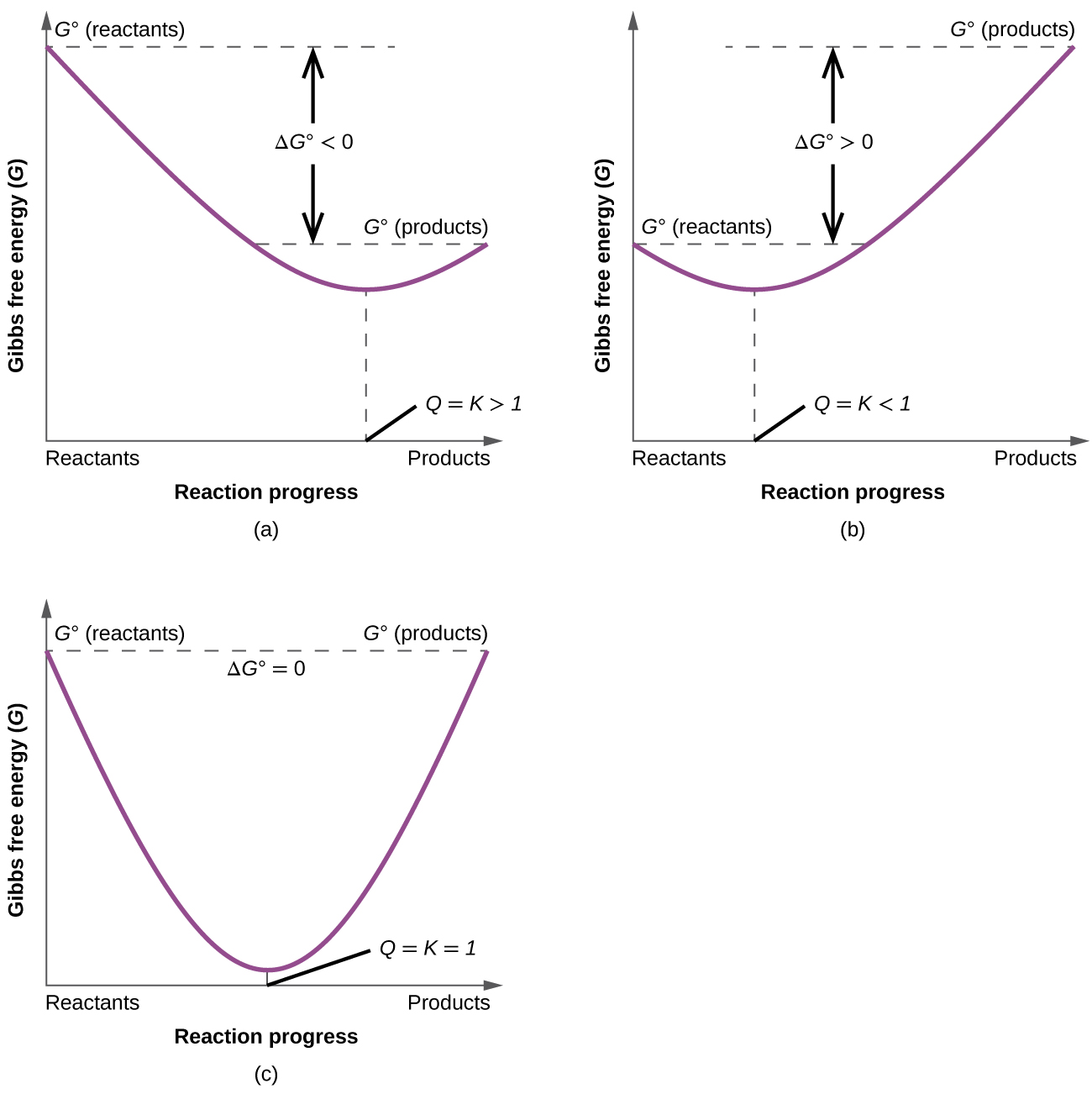
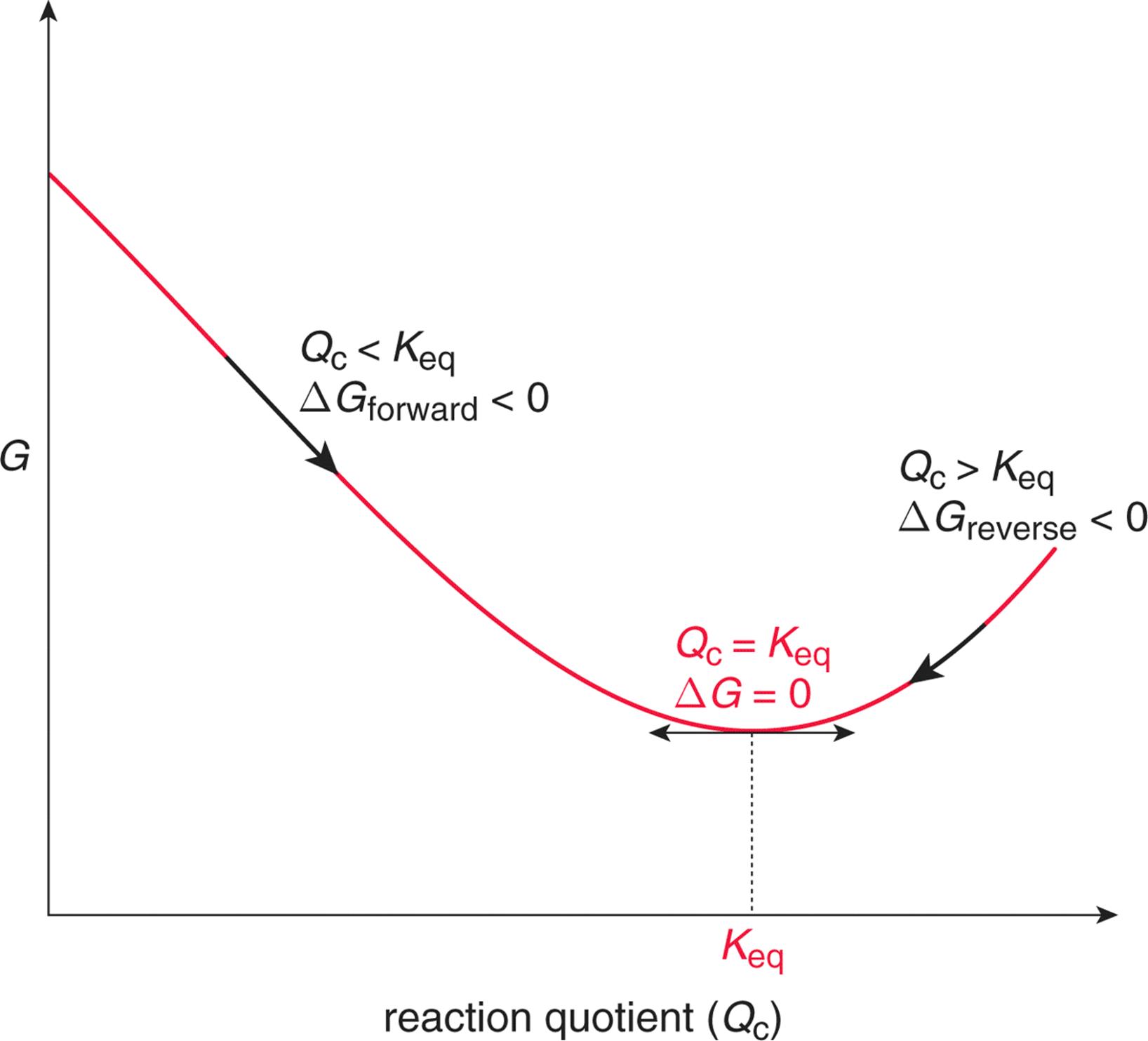
When a system is at equilibrium spontaneous. A spontaneous reaction is one that releases free energy and so the sign of ΔG must be negativeWhen ΔH is positive and ΔS is negative the sign of ΔG will always be positive and the reaction can never be spontaneousThis corresponds to both driving forces working against product formation. Recall that if K Q then the reaction proceeds spontaneously to the right as written resulting in the net conversion of reactants. If is positive the reaction is nonspontaneous it proceeds in the reverse direction.
Instead the reverse reaction is spontaneous. If is negative the reaction is spontaneous it proceeds in the forward direction. If the process is spontaneous ΔG 0.
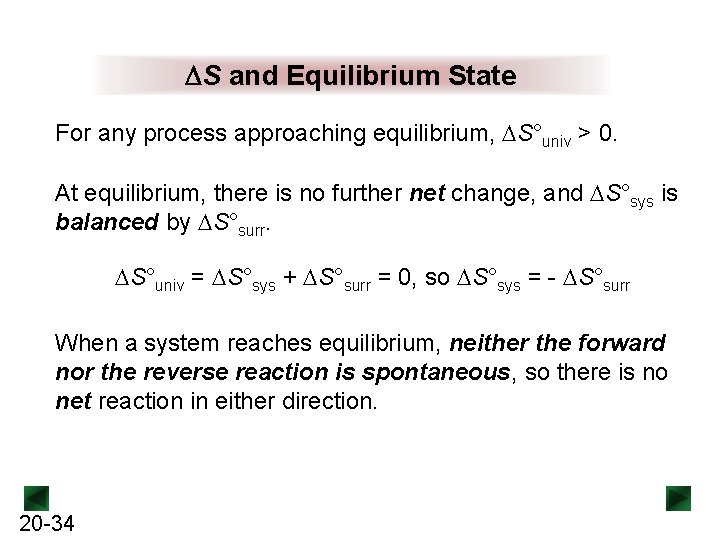
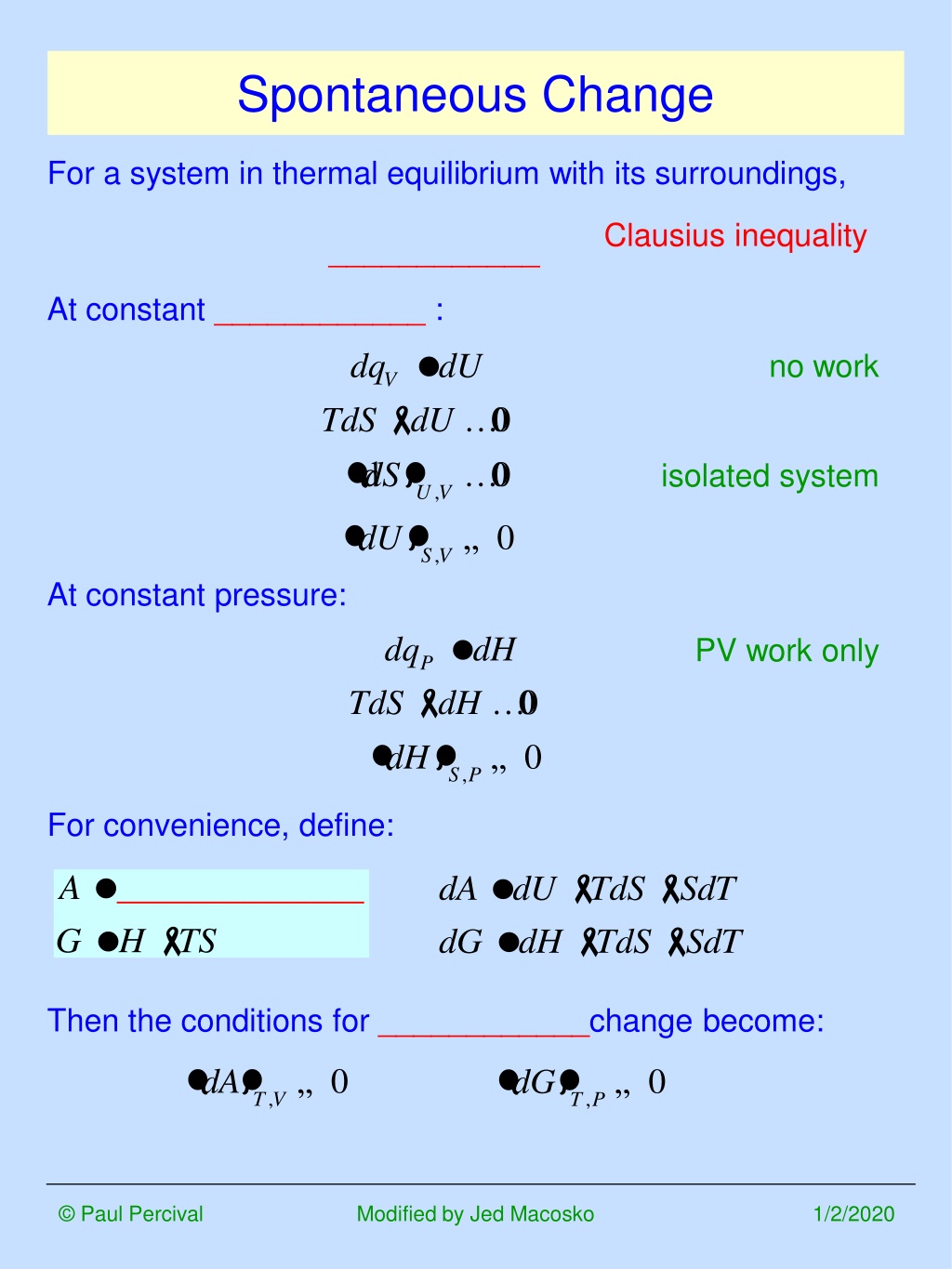
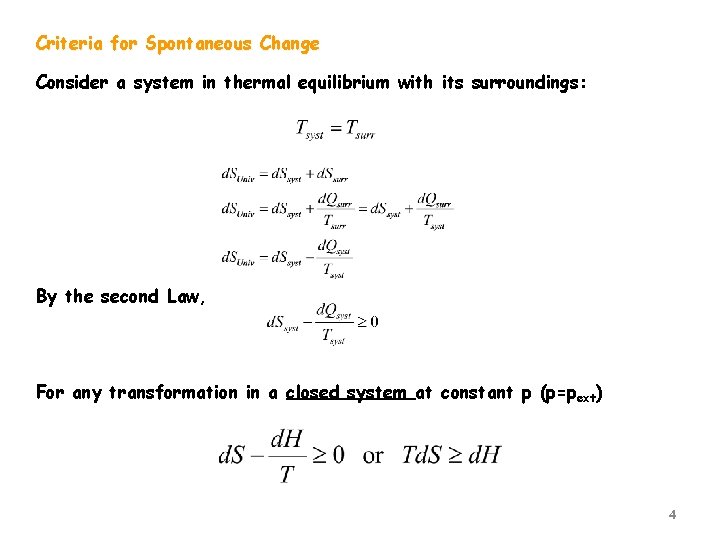
If heat leaves the system that heat increases the entropy of the surroundings. According to the second law of thermodynamics all spontaneous or natural processes produce an increase in entropy of the universe. C the forward and the reverse processes are both spontaneous.
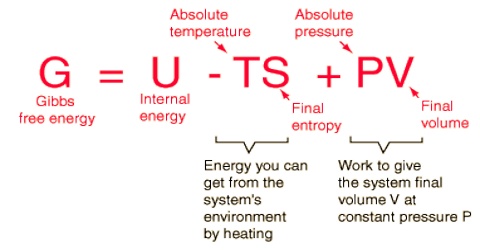
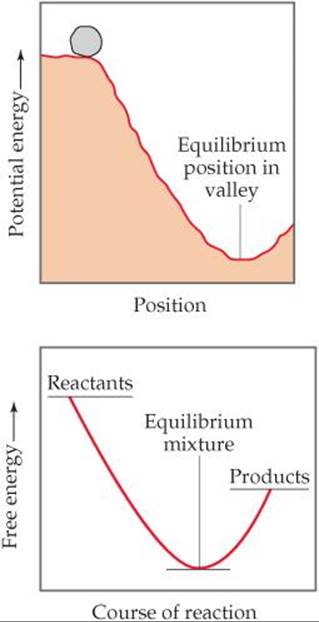
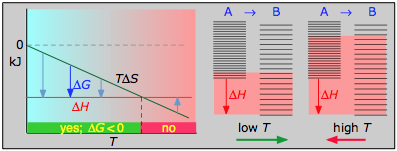
B the reverse process is spontaneous but the forward process is not. Why is entropy greatest at equilibrium. The relation between Gibbs energy and change in enthalpy is G H- T.
The process is not spontaneous in either direction. The Second Law of Thermodynamics states that in an isolated system any spontaneous change results in an increase in entropy. Any change can be easily detected in the spontaneous process.
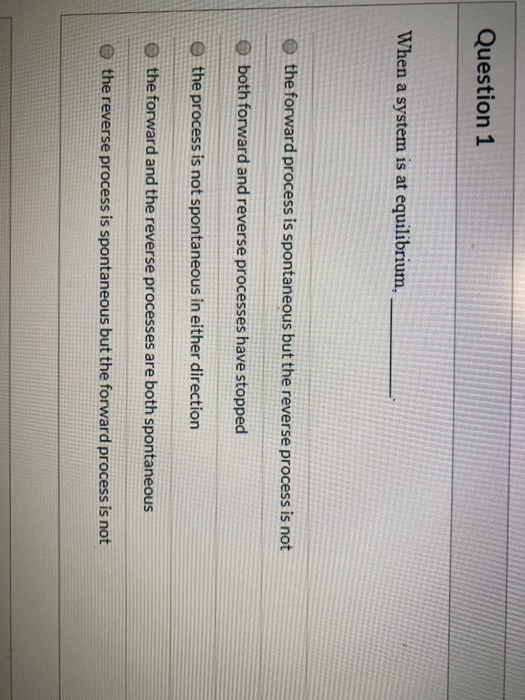
C If ΔS is negative then ΔH must be negative for a spontaneous process. If the process is not spontaneous as written but is spontaneous in the reverse direction ΔG 0. When a system is at equilibrium_____.
Both forward and reverse processes have stopped. When a system is at equilibrium________.
C the forward and the reverse processes are both spontaneous.
C If ΔS is negative then ΔH must be negative for a spontaneous process. Free Energy and Equilibrium If G o is negative or K 1 the reaction is spontaneous-Reaction occurs by just combining the reactants If G o is positive or K 1 the reaction is not spontaneous-Reaction requires external energy or process to proceed Gas flows towards a vacuum. 96 65 ratings Problem Details. C If ΔS is negative then ΔH must be negative for a spontaneous process. Spontaneous A vacuum does not naturally form. Equilibrium occurs when the rate of the forward reaction is equal to the rate of the reverse reaction. ΔH ΔG and ΔS refer to system changes a ΔS must be positive for a process to be spontaneous. Conversely if QK then the reaction proceeds spontaneously to the left as written resulting in the net conversion of products to reactants. What is the relationship between Gibbs free energy and equilibrium constant.
B ΔG is always positive for nonspontaneous processes. We have identified three criteria for whether a given reaction will occur spontaneously that is proceed in the forward direction as written to reach equilibrium. The reverse process is spontaneous but the forward process is not. If the process is spontaneous ΔG 0. Gibbs free energy relates enthalpy entropy and temperature. 4 After this hypothetical reverse move both the system and its surroundings are returned to their previous states. When a system is at equilibrium_____.




























Post a Comment for "When A System Is At Equilibrium Spontaneous"